Which Best Describes the Current Model of an Atom
Answered Which describes the current model of the atom. The atom contains no particles that have a neutral charge.

Atom Rutherford S Nuclear Model Britannica
3 Get Other questions on the subject.

. The positive charge in an atom is concentrated in one location. It is not an exact representation of the atom so it is not very useful. Which of the following is the correct inert core notation for uranium.
As more scientists attempt to find holes in the current theory adjustments are made which make the theory stronger. It is so trusted that no new information could possibly cause a change to the model. Which best describes the current model of an atom.
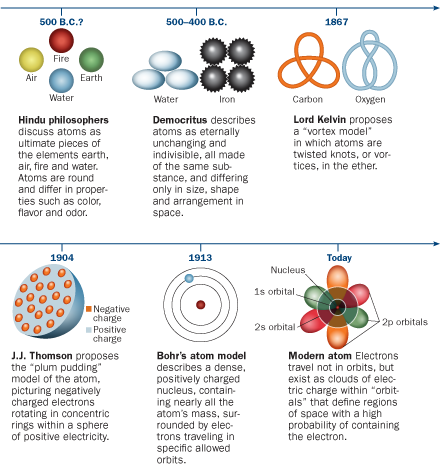
Answer choices a solid sphere with electrons and protons embedded a solid sphere unique for everything that exists a central nucleus containing protons and neutrons with electrons orbiting in levels of high probability a central nucleus containing protons with electrons orbiting in specific paths. C- it is similar to the model of plum pudding with suspended plums. My answer is C katrina Jan 9 2017.
Which best explains why scientific theories grow stronger over time. This current atomic model evolved from the earlier Rutherford-Bohr model which compared electrons orbiting an atomic nucleus to planets orbiting the sun. D- it may be revised with increasing scientific knowledge.
It is only accepted by a small fraction of scientists. B-it has remained the same for several centuries. Give two examples of what would occur if one of the pairs did not match or had an extra chromosomes.
Which best describes the current model of the atom. The newest understanding of atomic makeup in the Electron Cloud Model better. A-it is similar to the model of the solar system with orbiting planets.
It is only useful in very specific circumstances. Biology 22062019 0700 CheddaDsk. Rutherfords model introduced the nuclear model of an atom in which.
Which best describes the current model of the atom. The number of protons quals the number of electrons. Atoms are not divisible into smaller particles.
DProtons orbit the nucleus like planets orbit the sun. Which distinguishes an atom of one element from an atom of a different element. Which best describes the current atomic model.
Which best describes the relationship between subatomic particles in any neutral atom. Which best describes the current atomic theory. It is impossible to define the mass of an atom.
AThat electrons and protons move randomly around a nucleus. The model was the result of hundreds of years of experiments. How many protons are.
Which best describes the current model of an atom. Which best describes the current model of the atom. It is not an exact representation of the atom but is close enough to be very useful.
Explain how this might occur and if it would be dangerous to the individual. What describes the current model of the atom. Which best describes the current model of an atom.
CElectrons orbit the nucleus like planets orbit the sun. The number of protons how many protons are in an atom of bromine. An atom with which atomic diagram has chemical properties most similar to calcium.
Advertisement Answer 48 5 26 HnC1 A. The current model of atomic theory is called the Quantum Mechanical Model otherwise known as the Electron Cloud Model. Which best describes how the current scientific model of the atom was developed.
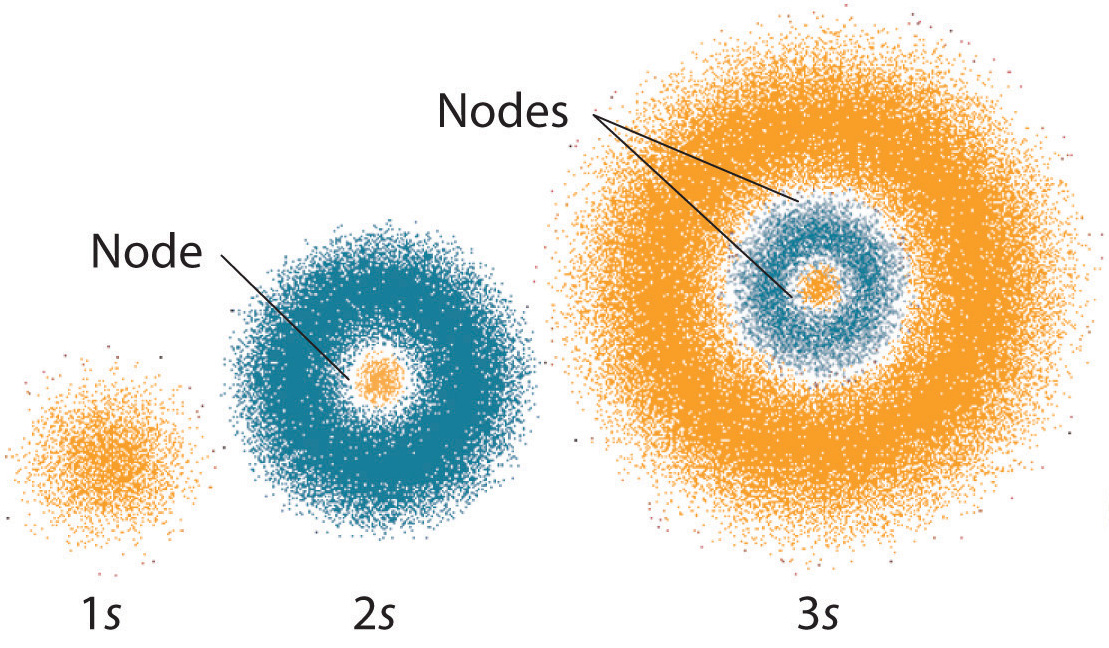
This was based upon the bright-line spectra of molecular hydrogen and lead to postulates that describe the electronic structure of the atom as having electrons in discrete energy levels and orbiting the nucleus much like planets orbit a star. It can be used to predict atomic behavior in most circumstances. A central nucleus with proton neutrons and electrons orbiting in levels of high probability which distinguishes an atom of one element from an atom of a different element.
BElectrons travel as waves in the electron cloud that surrounds the nucleus. A central nucleus contaiing protons and neutrons with electrons orbiting in levels of high probability. 35 which diagram shows an electrically neutral atom.
Why does the modern atomic theory. It describes exactly how the atom behaves in every circumstance. Atoms are composed of electrons in different clouds around a positive nucleus.
It has been perfected and will never be changed in the future. The number of protons.
The Quantum Mechanical Model Of The Atom Article Khan Academy
When The Atom Went Quantum Science News

Niels Bohr Atomic Theory And Its Limitations Atomic Theory Niels Bohr Chemistry Lessons
Comments
Post a Comment